The electron dot structure of carbon dioxide will be discussed through the following points
1. Electronic configuration of carbon
2. Electronic configuration of oxygen
3. Valence electrons in the carbon atom
4. Valence electrons in the oxygen atom
5. Bond between carbon and oxygen
6. Diagram of electron dot structure of `CO_2`
Electronic configuration of carbon atom
Carbon
has atomic number 6 and its configuration is
`1s^2
2s^2 2p^2`
Electronic configuration of the oxygen atom
Oxygen has atomic number 8 and its configuration
`1s^2
2s^2 2p^4`
Valence electrons in the carbon atom
Carbon
has four valence electrons (electrons in the outermost shell) and required four
electrons to complete an octet or stable noble gas configuration.
Valence electrons in the oxygen atom
The oxygen atom has 6 valence electrons and requires 2 electrons in the outermost shell to
complete a stable electronic configuration.
The bond between carbon and oxygen
One
carbon atom combines with two oxygen atoms. One carbon atom shares its
electrons with two oxygen atoms and forms a covalent bond (double bond).
Diagram of electron dot structure of `CO_2`
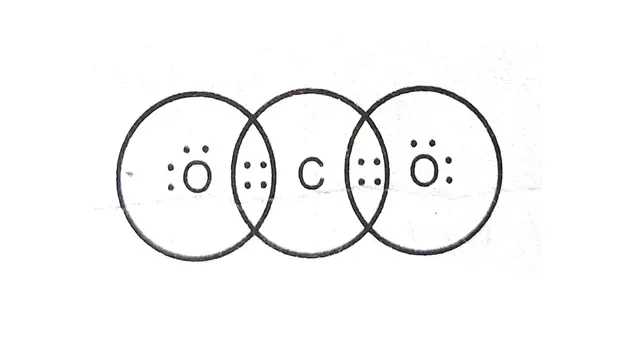 |
| Electron dot structure of CO2 |
Topics for you
3. Chemical Properties of Metals
4. Physical Properties of Metals
FAQs
1. How many valence electrons are
shared by carbon in `CO_2`?
Ans – Carbon contributes four valence
electrons with two oxygen atoms.
2. How many valence electrons are
shared by each oxygen atom in a `CO_2` molecule?
Ans
– Each oxygen atom contributes two electrons with carbon.
3. What is the geometry of carbon dioxide molecules?
Ans – Linear molecular geometry.
4. Are there any lone pair of electrons
in the Lewis structure of `CO_2`?
Ans – All valence electrons of carbon and
oxygen atoms are involved in forming a double bond.so there is no lone pair of electrons
in `CO_2`.
5. Which type of bond is present between carbon and oxygen atoms n carbon dioxide?
Ans – Double bond (covalent bond)
.jpg)

.webp)

No comments:
Post a Comment