In
this article, we are going to discuss the electron dot structure of `H_2S` which is also
known as the Lewis structure of `H_2S`.
We will discuss the electron dot structure of `H_2S` through
the following points
1. Formula of
Hydrogen sulphide
2. Electronic
configuration of hydrogen
3. Electronic
configuration of sulphur
4. Find the
central atom in the compound
5. Draw the
structural formula of hydrogen sulphide
6. Electron
dot structure of hydrogen sulphide
Formula of Hydrogen sulphide
`H_2S`
Electronic configuration of hydrogen
`1s^1`
Electronic configuration of sulphur
`1s^2 2s^2 2p^6 3s^2 3p^4`
Find the central atom in the compound
As the formula of hydrogen sulphide (Dihydrogen
sulphide) shows there is one sulphur atom connected to two hydrogen atoms, so sulphur
is the central atom.
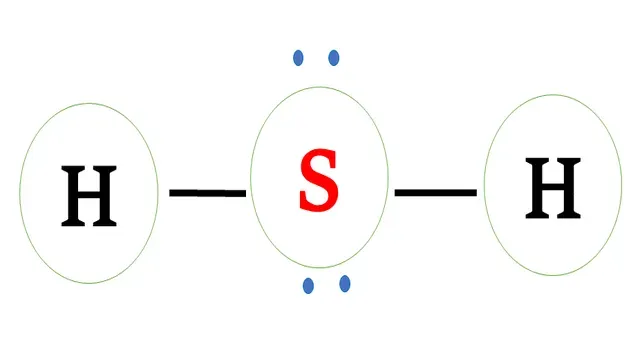
Structural formula of `H_2S`
Sulphur has six valence electrons and requires two electrons
to complete the octet.
Hydrogen has one valence electron and requires one
more electron to complete the octet.
Sulphur and each hydrogen share one -one electron to
form a covalent bond.
Electron dot structure of `H_2S`
Related Topics

.jpg)



No comments:
Post a Comment