Cyclopentane has a pentagonal structure. Today we will draw the electron dot structure of cyclopentane.
Electron Dot Structure of Cyclopentane
1. Formula of Cyclopentane
2. Electronic
configuration of carbon
3. Electronic
configuration of hydrogen
4. Find the
central atom in the compound
5. Draw the
structural formula of Cyclopentane
6. Electron
dot structure of Cyclopentane
Formula of Cyclopentane
`C_5H_10`
Electronic configuration of carbon
`1s^2 2s^2 2p^2`
Carbon has four valence electrons in the outermost shell.
Electronic configuration of hydrogen
`1s^1`
Hydrogen has one valence electron in the outermost
shell.
Find the central atom in the compound
Cyclopentane is a cyclic compound (pentagonal), so no
central atom is present.
Structural formula of Cyclopentane
Cyclopentane has five carbon atoms and
10 hydrogen atoms.
Each carbon atom is connected with two
carbon atoms and two hydrogen atoms.
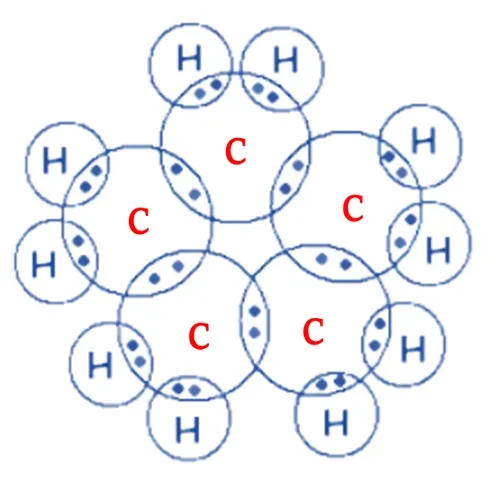
So, each carbon atom shares its four electrons
with 2 carbon atoms and 2 hydrogen atoms forming a single bond.
After sharing electrons, both carbon and
hydrogen complete their octet (stable electronic configuration).
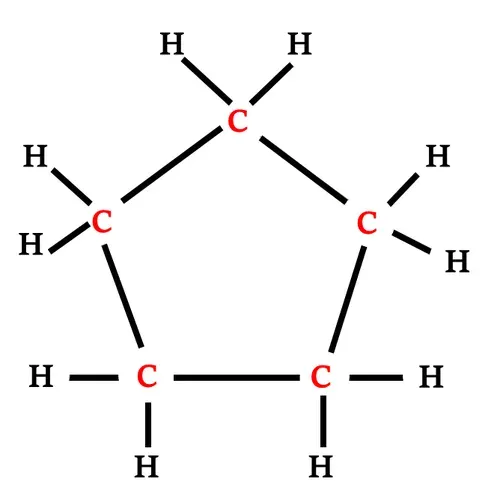 |
| Structural formula of Cyclopentane |
Electron dot structure of Cyclopentane
Related Topics



No comments:
Post a Comment