To draw the electron dot structure of s8, we must know the steps of electron dot structure or Lewis dot structures.
We will learn the electron dot structure of s8 molecule
through the following points
1. Formula of Sulphur
molecule
2. Electronic
configuration of Sulphur
4. Find the
central atom in the compound
5. Draw the
structural formula of s8 molecule
6. Electron
dot structure of s8 molecule
Formula of Sulphur molecule
Sulphur molecule is made of eight atoms of sulphur.
`S_8`
Electronic configuration of Sulphur
Sulphur has atomic number 16.
`[Ne]3s^2 3p^4`
Find the central atom in the compound
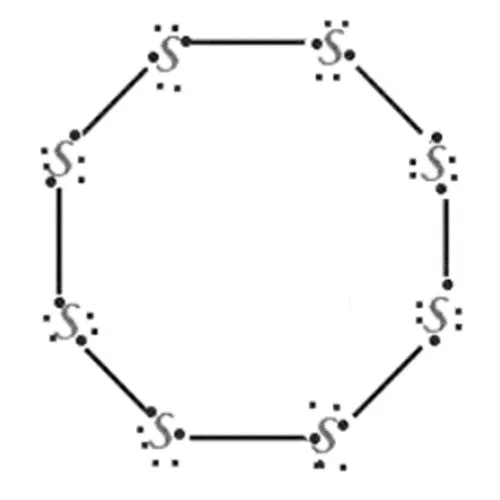
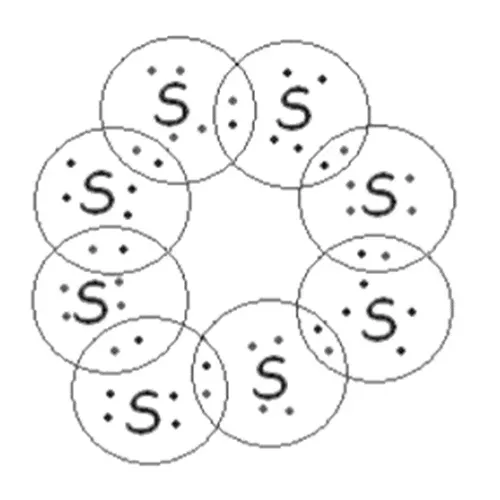
The S8 molecule has eight sulphur atoms and is joined in
the form of a ring.
Structural formula of s8 molecule
A sulphur atom has 6 valence electrons and needs 2
electrons to complete its octet or stable electronic configuration.
Each sulphur atom forms covalent bonds with two other
sulphur atoms by sharing two electrons and forming a ring-shaped structure.
Electron dot structure of s8 molecule
Related Topics
.jpg)

.webp)

No comments:
Post a Comment