Today we explore the chemical reaction that occurs when iron nails are placed in copper sulphate solution. This experiment is an example of a displacement reaction.
When the iron nails are placed in the copper
sulphate solution, a displacement reaction occurs and forms iron (II)
sulphate and metallic copper.
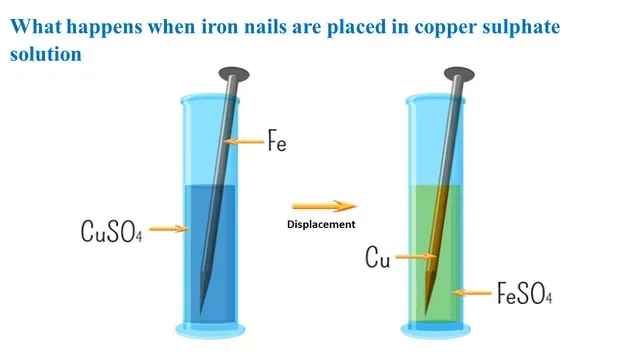 |
| Displacement Reaction |
The balanced chemical equation for the
reaction
Fe(s) + CuSO4(aq) → Cu(s) + FeSO4(aq)
Iron is more reactive than copper
so it displaces copper from the copper sulphate solution and form iron
sulphate and the blue colour of the copper sulphate solution fade as
the copper ions are removed from the solution.
At the same time, copper ions are reduced and deposited
onto the surface of the iron nails, forming a thin layer of copper on
iron nails.
In this reaction, the iron (Fe) is oxidized after losing electrons and iron ions (`Fe^(2+)`) are formed and remain in the
solution as iron(II) sulphate (FeSO4). the copper ions (`Cu^(2+)`) are reduced,
meaning they gain electrons. The copper ions are reduced to copper metal (Cu),
which is deposited onto the surface of the iron nail.
`Fe → Fe^2+ + 2e^-`
`CuSO_4 → Cu^(2+) + SO_4^(2-)`
`Fe^(2+) + SO_4^(2-) →FeSO_4`
`Cu^(2+) +2e^- → Cu`
So this is how the displacement reaction takes place when iron nails
are dipped in the copper sulphate solution.
Take a test to enhance your
knowledge
👇
1. What happens when magnesium ribbon is burnt?
2. What happens when Zinc reacts with sulphuric acid?
3. What happens when barium chloride reacts with sodium sulphate?
4. What happens when copper is heated in the air?
5. What happens when a potassium iodide solution is added to a solution of lead nitrate?
6. What happens when lead nitrate is heated?
7. What happens when silver chloride is exposed to sunlight?
FAQs
1. Which
chemical reaction takes place when iron nails are placed in copper sulphate
solution?
Ans. – Displacement reaction takes place when iron nails are placed
in a copper sulphate solution. Iron from iron nails displaces copper from copper sulphate
solution.
2. What
is the copper sulphate formula?
Ans.- CuSO4
3. What
is the iron sulphate formula?
Ans. – FeSO4
4. Why
does the colour of the solution change when iron nails are placed in copper sulphate
solution?
Ans.- Iron displaces
copper from the copper sulphate solution and form iron sulphate that’s why the blue
colour of the solution fades.
5. What
is the chemical reaction that occurs when iron nails are placed in copper
sulphate solution?
Ans. -
6. Can
iron nails dissolve in copper sulphate solution?
Ans.- No, iron nails
cannot dissolve in a copper sulphate solution. the iron nails undergo a
displacement reaction with the copper ions in the solution, where the copper
ions are reduced and deposited onto the surface of the iron nails, forming a
layer of copper on the iron nails. This process is called electroplating.
7. How
long does it take for the iron nails to become coated with copper when placed
in copper sulphate solution?
Ans.- This process can take a few hours to a few days for the
iron nails to become fully coated with copper. Mostly it depends upon some factors
like concentration of copper sulphate solution, amount of copper ions, temperature, the surface area of iron nails etc.
8. Can
the process of placing iron nails in copper sulphate solution be used for
electroplating?
Ans.- Yes, the process of placing iron nails in copper sulphate
solution can be used for electroplating. This process is known as
electrodeposition or electroplating. When a direct electric current is passed through
the copper sulphate solution containing the iron nails, the copper ions in the solution t deposit onto
the surface of the iron nails.
9 . Is
it safe to handle copper sulphate solution?
Ans.- copper sulphate is
a poisonous substance so one must carefully handle copper sulphate solution.



No comments:
Post a Comment