Today we will find the answer to what is displacement reaction. Definitions, types and examples are discussed below so that all your doubts will be removed.
Displacement Reaction Definition
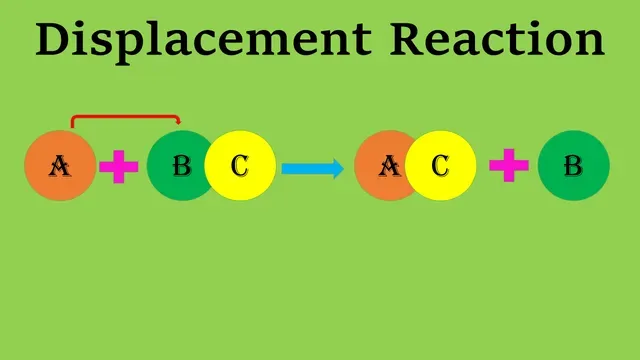
Displace = To remove
The reaction is called a displacement reaction in which one atom
or a group of atoms of one compound is displaced by one atom or a group of
atoms.
The reaction can be represented as follows:-
A + BC → AC + B
This reaction is of two types-
1. Single
Displacement Reaction
2. Double
Displacement Reaction
1. Single
Displacement Reaction: - A reaction in which a more reactive
element displaces a less reactive element from the solution of its compound, is
called single displacement or displacement.
`Zn + CuSO_4 → ZnSO_4 + Cu`
In the reaction, Zn is more reactive than Cu so Zn displaces Cu from copper sulphate.
2. Double
Displacement Reaction: - The reaction in which two different
ions in the reactant molecular are displaced by each other, is called a double
displacement reaction.
`Na_2SO_4 + BaCl_2 → BaSO_4
+ 2NaCl`
When aqueous solutions of
sodium sulphate and barium chloride are mixed slowly, an insoluble solid which is
barium sulphate a white precipitate
So, we can see in a double displacement reaction,
there is a mutual exchange of ions between reactants.
Single Displacement Reaction Examples
Following are the examples of single displacement reaction
1. Displacement
of copper from copper sulphate by Zinc
`Zn + CuSO_4 → ZnSO_4
+ Cu`
2. Displacement
of copper from copper chloride by lead
`Pb + CuCl_2 → PbCl_2
+ Cu`
3. Displacement of hydrogen from hydrochloric acid
by zinc
`Zn + 2HCl → ZnCl_2 + H_2`
4. Displacement
of lead from lead nitrate solution by iron
`Fe + Pb(NO_3)_2 → Fe(NO_3)_2
+ Pb`
5. Displacement
of hydrogen from water by Sodium
`2Na + 2H_2O →
2NaOH + H_2`
6. Displacement
of copper from copper chloride by magnesium.
`Mg + CuCl_2
→MgCl_2 + Cu`
7. Displacement
of silver from silver nitrate by Copper
`Cu + 2AgNO_3 → Cu (NO_3)_2 + 2Ag`
8. Displacement
of hydrogen from sulphuric acid by Aluminium
`2Al + 3H_2SO4 → Al_2(SO_4)_3 + 3H_2`
9. Displacementof copper from copper sulphate by Iron
`Fe + CuSO_4 → FeSO_4 + Cu`
10. Displacement of hydrogen from
hydrochloric acid by Magnesium
`Mg + 2HCl → MgCl_2 + H_2`
In all these chemical equations, one element
displaces another element from one compound.
Double Displacement Reaction Examples
Following are the examples of double displacement reaction
1. Barium
chloride reacts with sulphuric acid to produce HCl and `BaSO_4`.
`BaCl_2 +
H_2SO_4 →BaSO_4 +2HCl`
2. Reaction
between Sodium chloride and silver nitrate
`NaCl + AgNO_3 →
NaNO_3 + AgCl`
3. Reaction
between iron sulphide and dil. Sulphuric
acid
`FeS + H_2SO_4 →FeSO_4 +
H_2S`
4. Reaction
between barium chloride and sodium sulphate
`BaCl_2 + Na_2SO_4 →
BaSO_4 + 2NaCl`
5. Reaction between copper sulphate and ammonium hydroxide
CuSO4 + 2 NH4OH → Cu(OH)2 + (NH4)2SO4
6. Reaction
between potassium hydroxide and hydrochloric acid
`KOH + HCl → KCl + H_2O`
7. Reaction
between aluminium chloride and ammonium hydroxide
`AlCl_3 + 3NH_4(OH)→ Al(OH)_3 +
3NH_4Cl`
8. Reaction
between calcium carbonate and hydrochloric acid
`CaCO_3 + 2HCl →
CaCl_2 + CO_2 + H_2O`
9. Reaction
between Lead (II) nitrate r with potassium iodide
`Pb(NO_3)_2
+ 2KI → PbI_2 + 2KNO_3`
10. Reaction between Calcium chloride with
sodium carbonate
`CaCl_2 + Na_2CO_3 → CaCO_3 + 2NaCl`
So, the above chemical equations are examples of double
displacement reactions.
Related Topics
1. What happens during a chemical reaction
FAQs
1. Can
displacement reactions occur between non-metal elements?
Ans.- Yes, these reactions
can occur between non-metal elements.
`2KI + Cl_2
→ 2KCl + I_2`
2. Are
displacement reactions reversible?
Ans. – No, displacement reactions are irreversible.
3. Define
displacement reaction with an example.
Ans.- A displacement reaction is a chemical reaction in which one element displaces another element in a compound or a mutual exchange of ions between compounds.
`Zn + 2HCl → ZnCl_2 + H_2`
4. Are
displacement reactions exothermic?
Ans.- Displacement reactions can be either exothermic or
endothermic.
.png)

.jpg)

No comments:
Post a Comment