Butene is an unsaturated hydrocarbon and belongs to the Alkene series. In this article, we will discuss the electron dot structure of butene.
We will discuss the electron dot structure of butene through
the following points
1. Formula
of butene
2. Electronic
configuration of carbon
3. Electronic
configuration of hydrogen
4. Find
the central atom in the compound
5. Draw
the structural formula of butene
6. Electron
dot structure of butene
Formula of butene
`C_4H_8`
Electronic configuration of carbon
`1s^2 2s^2 2p^2`
Carbon has four valence electrons in the outermost
shell.
Electronic configuration of hydrogen
`1s^1`
Hydrogen has one valence electron in the outermost
shell.
Find the central atom in the compound
Butene is an unsaturated hydrocarbon. Carbon atoms are
joined to form the main chain.
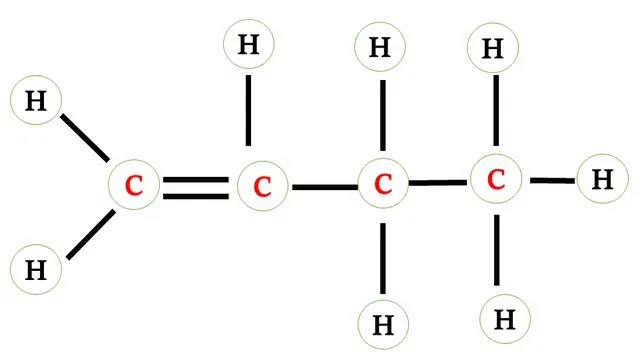
Structural formula of butene
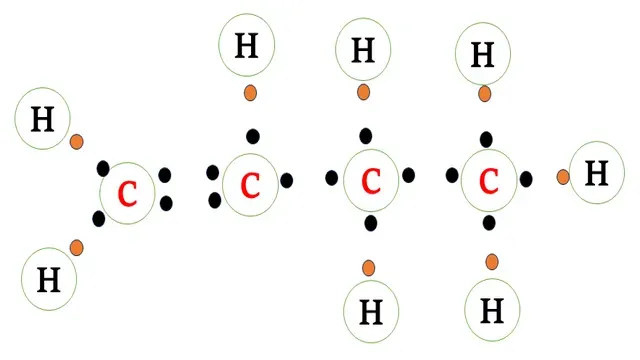
The carbon atom requires four electrons to complete its
octet and the hydrogen atom requires one more electron to attain a stable noble gas
configuration.
There is a double bond between any two
carbon atoms.
Two carbon atoms share 2 -2 electrons forming a double
bond.
The other bonds present between carbon atoms and carbon
and hydrogen atoms are single bonds that are formed after sharing 1-1 electrons.
Electron dot structure of butene(`C_4H_8`)
Related Topics

.jpg)


No comments:
Post a Comment