In this topic, we will discuss the electron dot structure of `NH_3` under the following points or steps.
In the series of electron dot structures, we are
discussing electron dot structures of different compounds.
1. Formula
of ammonia
2. Electronic
configuration of nitrogen
3. Electronic
configuration of hydrogen
4. Find
the central atom in the compound
5. Draw
the structural formula of Ammonia
6. Electron dot structure of Ammonia
6.
Formula of ammonia
`NH_3`
Electronic configuration of nitrogen
The atomic number of Nitrogen is 7
`1s^2 2s^2 2p^3`
Nitrogen has 5 valence electrons in the outermost shell
and it requires 3 electrons to complete its Octet.
Electronic configuration of hydrogen
`1s^1`
Hydrogen has 1 valence electron in the outermost shell
(only one shell) and requires one electron to complete an octet (stable electronic
configuration).
Three hydrogen atoms are present in ammonia.
Find the central atom in the compound
One Nitrogen atom combines with three hydrogen atoms, so Nitrogen is the central
atom in ammonia.
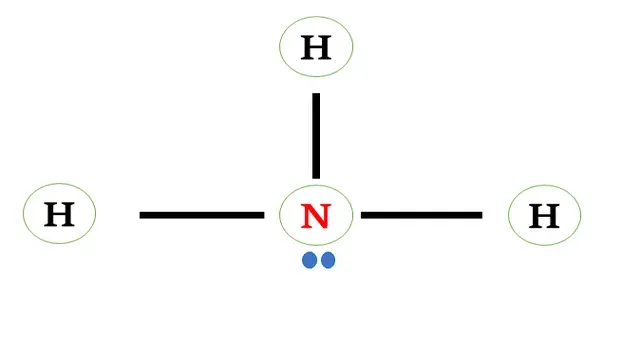
Structural
formula of ammonia
Now we have found that Nitrogen is the central atom of
ammonia.
Nitrogen shares three electrons with three
hydrogen atoms forming a single bond between them.
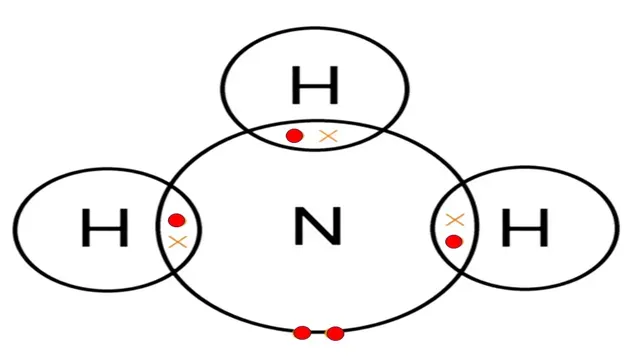
Electron dot structure of `NH_3`
Nitrogen has one lone pair of electrons (unshared
electrons).
So, this is the electron dot structure of ammonia class 10
which is very useful for students of class 10 onwards.
Related Topics
1. Electron dot structure of `CO_2`
2. Electron dot structure of ethanoic acid
.jpg)

.png)

No comments:
Post a Comment