Water is a polar solvent and the bonds between oxygen and hydrogen atoms are polar covalent. We will learn the electron dot structure of water.
The electron dot structure of water will be discussed
through the following points.
1. Formula of water
2. Electronic
configuration of oxygen
3. Electronic
configuration of hydrogen
4. Find the central atom in the compound
5. Draw the
structural formula of water
6. Electron
dot structure of water
Formula of water
`H_2O`
Electronic configuration of oxygen
`1s^2 2s^2 2p^4`
Electronic configuration of hydrogen
`1s^1`
Find the central atom in the compound
As the formula of water shows, there is one Oxygen atom
connected to two hydrogen atoms, so oxygen is the central atom.
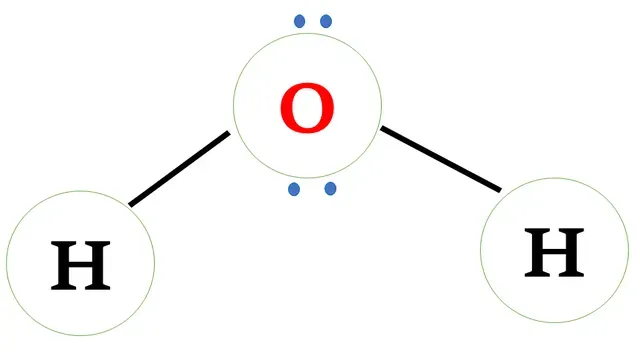
Structural formula of water
Oxygen has six valence electrons and requires two electrons
to complete the octet.
Hydrogen has one valence electron and requires one
more electron to complete the octet.
Oxygen and hydrogen share one -one electron to form a covalent bond.
Oxygen is an electronegative element so it attracts
shared electrons to a greater extent than hydrogen atoms.
The oxygen atom possesses a partial negative charge(`\delta-`)
Due to this bond between oxygen and hydrogen atoms
bends.
Following is the water molecule structure
Electron dot structure of H2O
Related Topics




No comments:
Post a Comment