In this post, you will find NCERT Science Class 9 Chapter 1 Activity solutions. In Class 9 science chapter 1 matter in our surroundings consists of NCERT Activity 1.2 Class 9 Science Explanation with a conclusion. You can also go through Class 9 Maths Chapter 6- Lines and Angles.
NCERT Activity 1.2 Class 9 Science Explanation with a Conclusion
You are suggested to study NCERT activity 1.2 class 9
science explanation with a conclusion so that you can attempt questions based on activity 1.2 class 9 science.
Activity 1.2 Class 9 Science
·
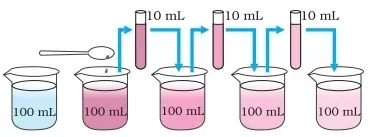
Take 2-3 crystals of potassium permanganate and dissolve them in 100 ml
of water.
·
Take out approximately 10 ml of this solution and put it into 90 ml of
clear water.
·
Take out 10 ml of this solution and put it into another 90 ml of clear
water.
·
Keep diluting the solution like these 5 to 8 times
·
Is the water still coloured?
 |
| Matter is made up of small particles |
Observation
when we add potassium permanganate into the water, water becomes pink or
purple but on dilution, the intensity of colour decreases.
Explanation
Crystal of potassium permanganate break down into smaller particles and
these particles spread out into the water. The colour of the water becomes pink or purple.
On dilution, the colour of the solution in beaker 2, beaker 3 or beaker 4 is
pink or purple but the intensity of colour becomes weak and the solution becomes
lighter and lighter.
Conclusion
After observing
this activity, we can conclude that each particle or piece of potassium permanganate
is made up of a number of small particles like atoms.
On diluting
these particles get divided and spread out in the solution, so a few crystals
of KMnO4 can make water pink or purple.
so it means one
small crystal of potassium permanganate has millions of tiny particles.
Questions based on activity 1.2 Class 9 Science
1. Write the formula of Potassium permanganate?
Ans .- KMnO4
2. What is the colour of the solution of potassium permanganate?
Ans. – Pink or purple
3. What does this activity indicate?
Ans.- This activity indicates that each substance is made up of small particles and these particles are called atoms.
4. Is the water in the last beaker coloured?
Ans. -Yes, the water in the last beaker colourd but the intensity of the colour is weak.
Related Topics
1. Activity 1.3 Class 9 Science
2. Activity 1.1 Class 9 Science
.png)


